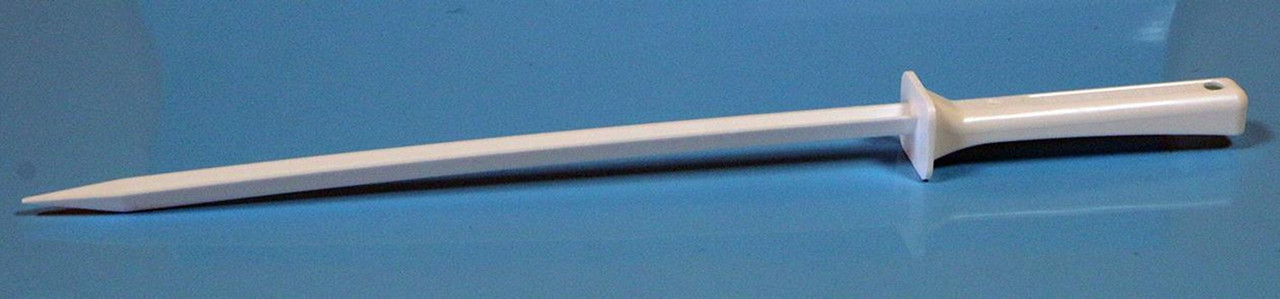
Product Description
SALES (804) 318-3686
QTY 100 Disposable Narrow V-Spatula Closed Tip 250 mm
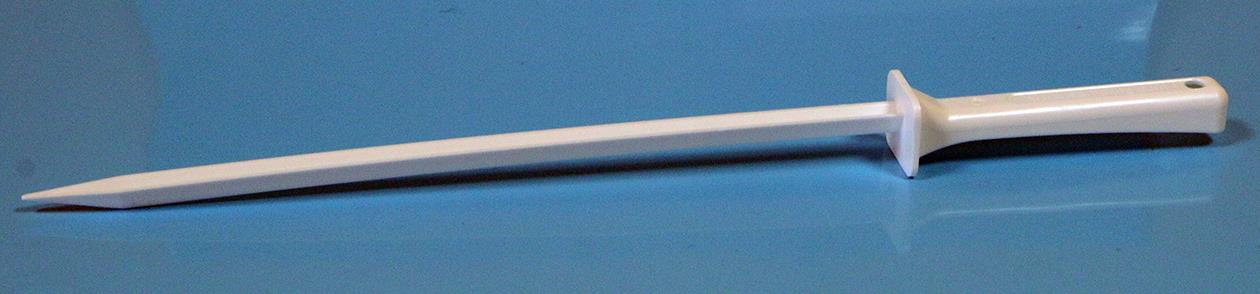
SPECIFICATIONS
Description: Disposable V-Spatula Closed Tip 250mm
Use:Single use, powder spatula
Material of Construction: Polystyrene - virgin
•Conforms to FDA CFR 177.1640
•Conforms to EU Regulations 10/2011
•Conforms to EC Regulations 1935/2004
•BSE/TSE free
Method of Construction: Single piece, injection molded
Blade Shape: V
Blade Style: Closed Tip
Overall Length 348 mm
Blade Length: 247 mm
Blade Width: 10 mm
Nominal Weight: 14 g
Nominal Volume (flat-filled): 4 ml
Moulding Environment: Class 100,000 Cleanroom
Primary Packing Environment: Class 10,000 Cleanroom
Individually Bagged? Yes (heat sealed PE/PET bag)
Method of Sterilisation: N/A
Number of Samplers per Box: 100
Recommended Storage Conditions: Dry and ambient temperature
Shelf Life: 5 years from the date of manufacture
ATEX: This device has not been ATEX tested
Enhance your sampling accuracy with the QTY 100 Disposable Narrow V-Spatula Closed Tip 250 mm, specifically designed for precise powder handling in laboratory environments.
Manufactured from virgin polystyrene, these spatulas ensure compliance with stringent regulatory standards, making them ideal for use in pharmaceuticals and other critical applications. The closed tip design minimizes contamination risks, guaranteeing purity in sampling.
- Single-use convenience to prevent cross-contamination
- Constructed from virgin polystyrene, ensuring compliance with FDA CFR 177.1640 and EU Regulations 10/2011
- Class 100,000 cleanroom manufacturing environment for high-quality integrity
- Individually bagged in heat sealed PE/PET bags for maximum hygiene
- V-blade shape allows for optimal powder collection and transfer
- Lightweight design (14 g) with a nominal volume of 4 ml for ease of handling
- Long shelf life of up to 5 years from the date of manufacture for reliable stock management
Experience precision and safety in your sampling tasks with the QTY 100 Disposable Narrow V-Spatula, ensuring your laboratory processes remain efficient and compliant.